reEvolution
PORPHYRINS
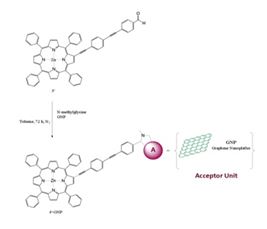
The project is based on the integration of graphene with different tetrapyrrolic metallomacrocycles through a covalent anchoring bond. These hybrids will be built on the basal planes of graphene, constituting a new material for improved sensors. Such improvement lies not only in the high sensitivity, low-cost and low-energy consumption, characteristic of graphene, but also in the specific response of the molecular moiety to relevant atmospheric pollutants. Specificity to adsorbates can be reached by introducing different metals and substituents in the macrocycle, for a fine tailoring of the molecular electronic properties, in relation to substrate and adsorbates.
Several chemical methodologies, based on well-established routes, have been developed to modify graphene. Cycloadditions, free-radical additions, and click reactions are the most frequently used when the basal plane of graphene is to be covalently functionalized. Cycloadditions of porphyrins and other tetrapyrrole macrocycles to graphene is an extension of Prato and coll. 1,3-dipolar cycloadditions, first proposed for fullerenes.
According to this, both electrical and optical features, exhibited by graphene and graphene derivatives, have been widely applied for detection of dangerous pollutants, providing an ideal and innovative sensing material for the assembly of highly sensitive environmental devices.
A wide application of porphyrins and phtalocyanines has been made for sensoring, but while use of graphene is relatively recent, the assembling of sensors by means of covalent links is, to date, lacking in the literature, as testified by the most recent reviews and articles.
